Our Hombourg site is Solvias’ Center of Excellence offering leading expertise in cell-based bioassays, molecular biology, and microbiology. This cGMP-compliant facility supports small molecules, biologics, gene and cell therapies, and other novel modalities. We offer tailor-made assays designed to fit specific client molecules and modes of action, along with regulatory-aligned validation packages to support both clinical and commercial use.
Center of Excellence for Cell-Based Bioassays, Molecular Biology, and Microbiological Testing

Contact us for a complimentary consultation
Integrated Testing Expertise Across Key Modalities
Solvias scientists at our Hombourg site have extensive experience providing analytical services for a wide range of therapeutic modalities:
- Monoclonal Antibodies (mAbs)
- Antibody-Drug Conjugates (ADCs)
- Recombinant Proteins
- Synthetic Peptides
- Nanobodies
- Small Molecules
- Immune Checkpoints Therapies
- Cell and Gene Therapies
- Tumor Vaccines
Detailed Capabilities Overview
Dive deeper into our cell-based bioassay, microbiology, and molecular biology services supporting a full range of therapeutic needs.
Identity Testing: Cell-based Binding Assay, ELISA, Cell-based Reporter Assay, Enzyme Activity Assays
Potency Assays: Cell Viability & Proliferation, ADCC (Antibody-Dependent Cellular Cytotoxicity), Cytotoxicity Assay, Sickle Cell Assay
Safety Assays: Colony-Forming Cell (CFC) Assay, Colony-Forming Unit (CFU) Assay, Lentiviral Vector (LVV) Quantification Preparation, Safety Cytokine Independent Growth (SCIG) Assay
Cell Banking & Platform Technologies: Master Cell Bank (MCB), Working Cell Bank (WCB), Flow Cytometry-Based Analysis, Method Development, Qualification, and Validation
Sterile Products: Compendial Sterility Test, Mycoplasma Detection, Bioburden Testing, Media Fill Test (MFT) for Aseptic Process Validation, Biological Indicator Steam Sterilization
Pyrogens & Endotoxins: Bacterial Endotoxin Test (BET), Monocyte Activation Test (MAT)
Non-Sterile Products: Microbial Enumeration Test (MET), Total Aerobic Microbial Count (TAMC), Total Yeast and Mold Count (TYMC)
Environmental Monitoring: Microbial Identification, DNA Sequencing (MicroSeq®), Proteomic Profiling (MALDI-ToF), Growth Promotion Testing of Culture Media
Facility & Product Studies: Container Closure Integrity Testing (CCIT), Disinfectant Efficacy Validation, Cleaning Validation, Low Endotoxin Recovery (LER) Studies
CHO-Based Products: Mycoplasma Detection, Residual Host Cell Protein (HCP) and DNA
AAV Products: Vector Copy Number (VCN) Quantification, Residual Nucleic Acid Testing, Replication Competent AAV (rcAAV) Testing
Cell Therapy Products: Mycoplasma Detection, Cell Count Determination
LVV Products: VCN Quantification, Residual Nucleic Acid Testing, Gene of Interest (GOI) Expression Profiling
CAR-T Products: Integrated VCN Quantification, GOI Expression
Raw Materials: Mycoplasma Detection, DNAse/RNAse Contamination Screening
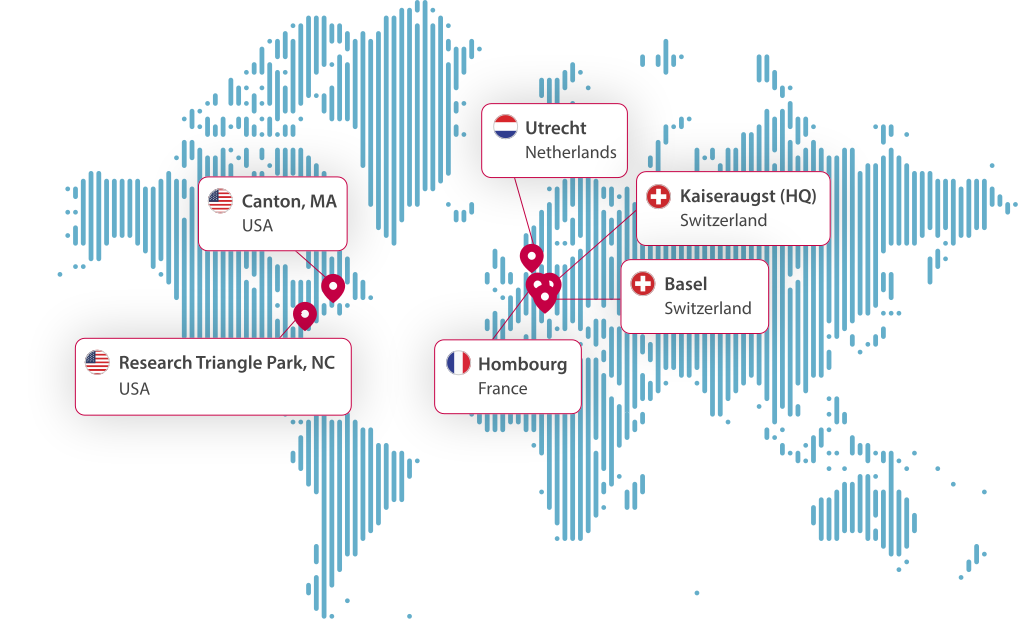
By the Numbers: Solvias’ Global Footprint
With six facilities worldwide, our operations span 25,000 m² of lab capacity, supported by 800+ employees. We serve over 175 customers globally, including 19 of the top 20 pharmaceutical companies. Our locations include Canton (USA), Kaiseraugst (HQ) and Basel (Switzerland), Utrecht (The Netherlands), Hombourg (France), and Research Triangle Park (USA).
What Our Clients Say
“Solvias’ scientists are highly trained, innovative, and business-minded. Their analytical solutions are outstanding.”
“Their project managers are flexible, responsive, and committed to our success.”
“Solvias is not just a vendor—they are an extension of our team.”